RGCC Cancer Test & Therapies
Our team at RGCC Malaysia is driven by a profound commitment to advancing the field of personalised cancer care. Our goal is simple yet powerful: to empower people facing cancer with tailored insights that can change the course of their journey.

About Us
RGCC was launched in 2004 by genetics pioneer Dr. Ioannis Papasotiriou who believes that the key to effectively treating cancer lies in personalized medicine using the information in a patient’s genes.
Using world-leading technology, equipment and innovative techniques, our team of scientists have developed a range of tests that give healthcare professionals comprehensive information about a patient’s genetics, physiology and immune profiles. This diversion from a ‘one-size-fits all approach’ uses data to determine what treatment will work best.
Most importantly, by looking at a person’s genetics, physiology and immune profiles, our cost-effective and highly accurate tests will give you the information you need to formulate effective personalized treatment plans.
Our laboratories in Europe are equipped with world-leading technology and staffed by experts in their fields. Using innovative techniques, they provide trusted and timely information so doctors and patients can take action based on their results.
ACCREDITATIONS






Meet Our Founder

Dr. Ioannis Papasotiriou
MD, PHD | Managing Director at Research Genetic Cancer Centre International GmbH
In 2004, genetics pioneer Dr. Ioannis Papasotiriou founded RGCC with a steadfast commitment to personalized cancer care. Driven by his unwavering belief in the power of a patient’s genes, Dr. Papasotiriou has passionately refused to access external funding that could compromise his vision and commitment to a science-driven cancer solution.
His dedication and expertise have enabled RGCC to stand at the forefront of cancer treatment, providing hope and healing to patients and their families around the world.
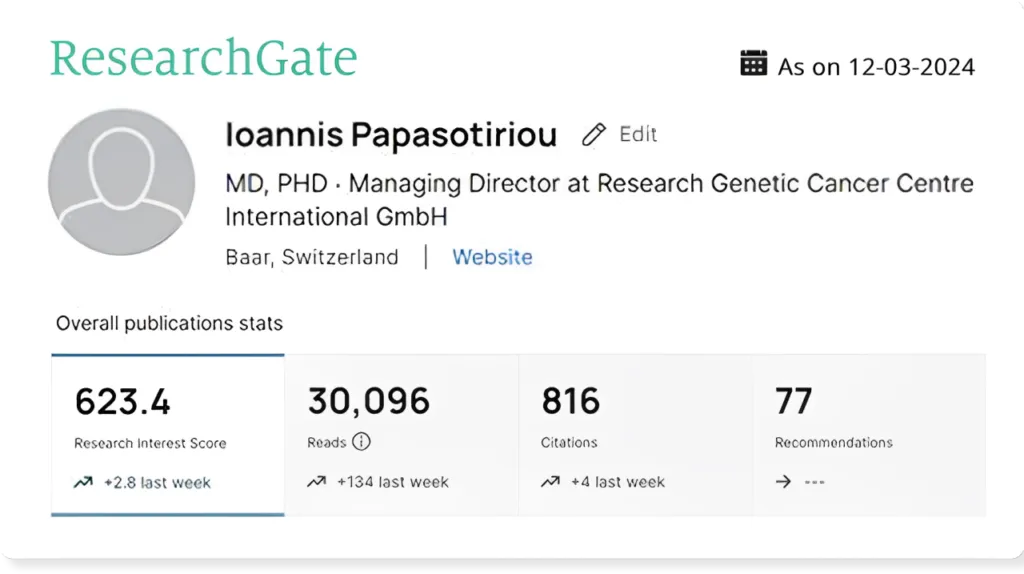
Our founder’s research gate analytics serves as a testament to the R&D and patient centric approach that is core of what we do at RGCC.

RGCC Tests
We have developed a range of cutting-edge tests designed to help discover, analyze and screen cancer cells at every step of the disease.
RGCC’s objective is to equip patients and clinicians with the most robust, reliable and accurate information about cancer so they can choose the treatments that will work best.
1. Onco-D-clare
Onco-D-clare is a cancer screening test based on the interaction of molecular biology with machine learning algorithms. This screening test can help detect cancer before symptoms appear. Peripheral blood mononuclear cells are isolated from blood samples, and gene expression analysis is performed in more than 90 genes. Their expression profile is then used for the classification of the sample as cancerous or healthy. The Onco-D-clare test provides individuals with essential information to help them make better-informed decisions about future health and well-being. The test is clinically validated and highly accurate (≈93%).
2. InVyomma Plus
InVyomma Plus focuses on gut microbiome profiling, including analysis of short-chain fatty acids, and how they are associated with cancer.
It has been discovered that the microbiome is closely related to the occurrence and development of a variety of cancer types in the epithelial barrier and sterile tissues. Symbiotic ecosystems that inhabit the gut or other mucosa perform a role in both local and distal carcinogenesis.
On the one hand, the microbiome can provide toxic metabolites or carcinogenic products directly as cancer-transforming agents. Moreover, it can indirectly play a role in promoting cancer by the induction of inflammation or immunosuppression and can modulate the efficacy and toxicity of cancer therapy.
The InVyomma Plus test provides clinicians with essential information to help them make better-informed decisions about future treatment, health, and well-being.
InVyomma Plus requires 4 stool Collection Tubes, 2 for the gut microbiome profiling, and 2 for short-chain fatty acids analysis. The experimental/analysis period is approximately 2-3 weeks, and the results are ready in 2-4 weeks after the sample’s arrival in the laboratory facilities.
3. Onconomics Extracts+
Onconomics Extracts+ provides highly detailed and accurate information about how effective specific natural substances and plant extracts are, as anticancer agents as well as the gene expression profile of genes, associated with fundamental biological processes. This includes processes such as angiogenesis, cell cycle regulation, self-repair, metastasis, apoptosis, proliferation etc.
Circulating tumor cells are isolated from a peripheral blood sample and then used for different assays. The results provide a comprehensive and highly personalized analysis of the most effective natural treatments that patients can use to treat their cancer, alongside conventional therapies.
Natural substances and extracts that are tested are divided into Class-I (Cytotoxic agents), Class-II (Immunostimulants / immunomodulators), and Class-III (PK inhibitors).
4. RGCC CAMBISeq®
RGCC CAMBISeq® is an innovative test that uses next-generation sequencing analysis on both DNA and RNA to provide clinicians with crucial insight into cancer. The CAMBISeq® – or cancer analysis, mutational burden and instability sequencing – test is used to identify variants in 500 genes that our scientists use as biomarkers to assess their sensitivity to immunotherapy. The results can predict how well a patient will respond to types of immunotherapy. RGCC CAMBISeq® is suitable for all patients who have a confirmed cancer diagnosis. The test can be used by clinicians to identify the best, and most effective, combinations of cancer treatments.
5. Array comparative genomic hybridisation (aCGH) RGCC
The array comparative genomic hybridisation (aCGH) RGCC test is used to identify chromosomal abnormalities in a patient that could lead to cancer.
During the test, scientists use a technique called array comparative genomic hybridisation to spot abnormalities in a genome. This insight enables them to assess the likely risk of cancer developing and the potential location of a primary tumour. aCGH RGCC can help clinicians to understand more about a patient’s genes, giving them a powerful tool in the fight against cancer.
6. Immune-Frame
Immune-Frame is used to assess the condition of a patient’s immune system. Scientists use the test to identify specific cellular markers that are responsible for switching a patient’s immune system on and off. The results of Immune-Frame can be used to analyze the status of a patient’s immune system, and to provide ongoing information about their health status. This information can be used by clinicians to advise on potential health risks a patient may face, and how these can be minimized.
7. Metastat RGCC
Metastat RGCC is an advanced test to detect specific blood-borne markers that can accurately determine whether a secondary cancerous tumour is likely to develop and its potential location. During the test, we analyze a sample of a patient’s blood in our state-of-the-art laboratory to analyze, identify and measure circulating tumour cells (CTCs). Metastat RGCC is suitable for all patients who have received a confirmed cancer diagnosis. The test can accurately detect the development of secondary cancers or tumours and improve personalized cancer treatments.
8. ChemoSNiP
ChemoSNiP is an innovative test that uses an advanced scientific technique called pharmacogenomics to analyze how a patient’s body will respond to a specific drug. ChemoSNiP analyses a blood sample to identify single nucleotide polymorphisms – variations in our DNA sequence that can affect if we develop cancer or if we respond to treatments with chemicals, drugs and other agents. ChemoSNiP provides clinicians with a powerful insight into which drugs are most effective at treating cancer. The results provide a personalized analysis that can be used to provide the most effective combination of drugs and treatments available.
9. Oncocount RGCC
Oncocount RGCC detects the presence of circulating tumour cells (CTCs) and their concentration in the blood. CTCs are a powerful biomarker, and their presence in the blood can act as an early warning sign that cancer is returning. The test is designed as a follow-up test for patients who have cancer and are worried about the disease returning. Clinicians can use the Oncocount RGCC test to assess how effective cancer treatment is and the likelihood of cancer returning (relapse).
10. Onconomics Extracts
Onconomics Extracts is a unique and highly detailed test that provides information on how effective natural substances and plant extracts are at attacking circulating cancer cells (CTCs). Onconomics Extracts uses three unique and scientifically-proven methods to assess how effective natural treatments are at tackling cancer. The results provide a comprehensive and highly personalized analysis of the most effective natural treatments that patients can use to treat their cancer, alongside conventional therapies.
11. Onconomics Plus RGCC
Onconomics Plus RGCC provides information about the effect of specific anti-cancer drugs, targeted therapies and natural treatments on the cancer cells in an individual patient. During the test, a sample of blood or tissue is analyzed to test how effective specific therapies and treatments are at suppressing cancer. Together, the results of the extensive tests provide scientists and clinicians with a comprehensive breakdown of the most suitable and successful treatments for cancer. The results can be used by clinicians to design personalized and targeted cancer therapies with the highest chances of success.
12. Onconomics RGCC
Onconomics RGCC provides highly detailed and accurate information about how effective specific anti-cancer drugs and targeted therapies are in treating cancer. The test combines a molecular and a cellular approach by incorporating two procedures: epigenetic analysis and viability assays. A sample of blood or tissue is analyzed to test how effective specific therapies and treatments are at suppressing cancer. Together, the results of these two testing methods provide scientists and clinicians with a comprehensive breakdown of the most suitable and successful treatments for cancer.
13. Oncotrail RGCC
Oncotrail RGCC provides crucial information on the presence of circulating tumour cells (CTCs) and their concentration in patients who have a confirmed diagnosis of specific forms of cancer, including breast, colon and prostate cancer. During the test, a sample of blood is analyzed to identify the presence and concentration of CTC and their immunophenotype. Oncotrail RGCC isn’t used as a primary diagnostic test to confirm a cancer diagnosis, but provides essential information on the effectiveness of current cancer treatments. Follow-up tests can also be used to monitor a patient’s health and assess the risk of relapse.
14. Oncotrace RGCC
Oncotrace RGCC is used to identify a primary tumour in a patient and to provide guidance about disease progression and future prognosis. During the test, a sample of blood is analyzed to identify the presence and concentration of circulating tumour cells (CTCs) and their concentration. This test provides information about the presence of CTCs, their concentration and their specific type (immunophenotype). The results of the test enable clinicians to identify the origin of a tumour where this is unknown and to provide information on the development of cancer and the prognosis.
RGCC Therapies
Treating chronic disease varies for each patient.
Our multidisciplinary team merges research with technology to develop personalized therapies, improving outcomes.
The field of advanced therapy medicinal products (ATMPs) has enormous potential to fulfil previously unmet medical needs. Our scientists are working to translate this potential into reality with a range of ATMPs to effectively treat a range of chronic diseases, including cancer.
1. SUPPORTIVE OLIGONUCLEOTIDE THERAPY (SOT)
SOT is a personalized therapy for cancer, viruses and pathogens including Lyme Disease. The SOT has the ability to bind to a specific gene of interest that controls the cancer cell or pathogen by disrupting the critical process that promotes its survival or growth. During this therapy, we analyze a patient’s blood and we create a complementary oligonucleotide sequence that is designed to interrupt survival or growth of the cells.
2. VAXO-Q-RE
Vaxo-Q-Re is a new advanced therapy consisting of five types of immune cells; macrophages, NK cells, dendritic cells, cytotoxic T lymphocytes and antibody producing plasma cells. Tested on breast cancer cells but effective at treating all forms of the disease, the adoptive T cell-based therapy encourages the body’s immune system to target cancer cells. Vaxo-Q-Re has been in development by RGCC scientists for more than five years and is now categorized as a Cell Therapy Medicinal Product (CTMP) by the European Medicines Agency (EMA).
Frequently Asked Questions
About RGCC
Where is RGCC based?
RGCC’s headquarters are in Switzerland. We have laboratory facilities in Greece, Germany and India. We work with a global network of healthcare providers across Europe, the USA and the rest of the world to offer our tests. We currently operate in 23 countries but are expanding our network.
Where will my test be performed?
Blood samples will be analyzed in one of our three facilities, in Greece, Germany or India. We are unable to tell you where your test will be processed. Our laboratories work to the highest standards and process tests in exactly the same way to ensure their validity.
What else does RGCC do?
RGCC is at the forefront of personalized genetic testing for cancer. Alongside this, we are actively involved in pharmaceutical research and development working from our world-leading facilities in Europe. Our scientists regularly publish peer-reviewed research and are respected across the world.
Can I speak to someone at RGCC?
Yes. You can use our online contact form, or visit our contact page where you can find ways to get in touch with us.
What does RGCC stand for?
RGCC stands for Research Genetics Cancer Center. We prefer RGCC.
RGCC Tests
What types of tests does RGCC offer?
RGCC has developed a range of tests to discover, analyze and screen cancer cells. We currently offer 11 tests that can detect potential early signs of cancer, monitor existing diagnosed cancers and provide personalized guidance on the most effective combination of cancer treatments and therapies, including complementary therapies.
I have symptoms of cancer. Do you offer a diagnostic test?
If you have any symptoms of cancer, you should speak to a qualified medical professional urgently. You can book a consultation with an RGCC Network clinician by sending us a message via our website. Alternatively, you can download and print this letter and take it along with you to your appointment.
How do I order a test through RGCC?
RGCC tests are only available to the RGCC Network of healthcare professionals. If you are a health professional interested in learning more about the RGCC Network, please contact your local branch office. If you are a patient interested in ordering an RGCC test, you can request one through your personal healthcare provider, or contact one of our global network of healthcare providers. To find your nearest RGCC registered clinician, send us a message via our website.
Why do I need to go through a clinician to order an RGCC test?
RGCC tests are designed to provide medical information and advice for clinicians. The information contained in the results needs to be interpreted by the clinician in charge of your care. If you are a patient interested in learning more about RGCC’s range of tests, you can find your nearest RGCC clinician by sending us a message via our website. Alternatively, you can download and print this letter and take it along with you to your appointment.
I’m not an RGCC Network clinician. Can I still order a test?
RGCC tests are only available to the RGCC Network of healthcare professionals. We are always looking to connect with individuals and organizations who share our values. If you are a health professional interested in learning more about the RGCC Network, please contact your local branch office
What happens when I order a test through RGCC?
Once you have ordered your RGCC test, you will need to provide us with a sample of blood. We will provide all shipping instructions to ensure the sample arrives in perfect condition. The sample is processed at one of our world-leading laboratories. Once the test is completed, we provide you with a detailed breakdown of what we have found. We recommend that you and your patient discuss your results and work together to develop a personalized treatment plan based on our findings. You can learn more about the RGCC test process by visiting the RGCC test pages or downloading our patient information leaflet.
Why aren’t RGCC tests offered by my healthcare provider?
RGCC’s tests are at the cutting-edge of medical science and are not currently offered by healthcare providers. Health providers, such as the NHS, are trialing genetic testing to diagnose and treat cancer. In the future, it is likely that genetic testing will be offered by all healthcare providers. At RGCC, we believe that everyone should be able to experience the benefits of personalized medicine. Our range of genetic tests is currently available in 23 countries through the RGCC Network of healthcare professionals. To find your nearest RGCC registered clinician, send us a message via our website.
Are RGCC’s tests accredited?
Our methods are accredited by the International Organization for Standardization (ISO). Details of our certifications can be found on our website.
Is my information safe?
Yes. We take considerable care in protecting the patient’s identity throughout the process. We have strict data protection policies in place and adhere to all legislation, including GDPR.
Will you use or resell my genetic information?
We never use or resell your genetic information to third parties. The sample you provide to use is only used for the intended test.
What happens to my blood sample after the test?
Once the test has been completed, your sample will be stored for a time period of six months, just in case any further testing will be required.
Medical & Technical
Is it possible to measure circulating tumour cells (CTC) in patients with lymphoma or leukaemia?
Yes, patients with lymphoma or leukaemia can use an RGCC test to measure the number of circulating tumour cells (CTC) they have.
Which test is the most comprehensive test?
The most comprehensive test is the “Onconomics Plus” test. This provides information on the sensitivity or resistance of the patient’s tumour cells to certain cancer drugs and shows options for targeted therapy or an alternative treatment method with organic substances. The test also contains information about the development of the tumour and its potential by identifying epigenetic tumour markers relevant for the therapy, which are crucial for the correct therapeutic approach.
Which cancer cells do you test in an RGCC test?
In any tumour there are several subpopulations of cancer cells, but we only harvest and analyze cells with the tumour-initiating properties.
How are the Onconomics Plus and ChemoSNiP tests different?
The Onconomics Plus test provides information about the sensitivity or resistance of specific anti-cancer drugs, targeted therapies and natural treatments on the cancer cells in an individual patient. The ChemoSNiP test can be used to predict whether a patient will respond to drugs and metabolize them properly.
In which countries are RGCC tests approved for use?
Analysis assays cannot be approved. The only test that is approved by the US Food and Drug Administration and the European Medicines Agency is the Cellsearch test. The way we analyze circulating tumour cells is certified for accuracy.
Are your testing methods accredited internationally?
Our methods are accredited by the International Organization for Standardization (ISO). Details of our certification can be found on our website.
What’s the scientific evidence for the tests RGCC provide?
You can find the data we have published and presented at medical conferences here.
I only have a blood sample. Is it still possible to order a test that requires both blood and tissue samples?
This depends on the cancer. With the exception of the Oncotrace test, every RGCC test can be conducted with just a blood sample. Certain cancers – for example, glioblastoma and other types of brain cancer – require both a blood and a tissue sample for tests.
How many types of cancer are tested when RGCC says “all types of cancer”?
These tests are for all solid and blood tumour malignancies, with the exception of primary tumours of the central nervous system.
Is it possible to test for circulating tumour cells in a tissue sample?
We calculate the number of circulating tumour cells from the total amount of blood cells, expressed as the volume of circulating tumour cells per millilitre of blood. This cannot be calculated from a tissue sample. To conduct a circulating tumour cell test, a blood sample must be provided.
Why is the circulating tumour cell count not indicated in tissue samples?
The tissue sample taken from the tumour during a biopsy is part of the tumour contain cancerous cells, so there is no need to search for CTC populations.
Can a circulating tumour cell test be carried out during chemotherapy?
Yes, but a patient must wait seven days after chemotherapy treatment before taking the test.
Which test is appropriate for gene expression?
The Onconomics and Onconomics Plus tests look for the expression of genes that are linked with the effect of specific drugs on the cancer cells in a single patient.
What do immunophenotype tests show?
The comparative genetic hybridization (CGH) test provides information about genetic abnormalities present in tumour cells that can be associated with tumours. This test is relatively new and we do not yet provide it at RGCC.
Which test focuses on the efficacy of therapeutic agents against a patient’s cancer?
The ChemoSNiP test can be used to predict whether a patient will have a good or bad response to a drug, or no response at all.
Which tests are recommended to identify active substances and drugs that work against a tumour?
We recommend the Onconomics or Onconomics Plus tests.
Which test focuses on the status of the immune system and immunity?
The Immune-Frame test focuses on the status of a patient’s immune system, based on their cellular markers and the level of cytokine proteins in the test sample.
Which tests are recommended for follow-ups and patient monitoring?
Oncocount, Oncotrace or Oncotrail tests can be used to follow up and monitor specific types of tumours.
Do circulating tumour cells predict the site of relapse and metastases?
Our Metastat test provides this information over a period of three years. It compares profiles of CTCs and metastases to detect relevant markers at the site of relapse.
Can patients with lymphoma or leukemia have their circulating tumour cell count measured?
Yes. Patients with lymphoma or leukemia can use an RGCC test to measure their circulating tumour cell count.
Can RGCC test for BRAF drugs?
Yes. Onconomics and Onconomics Plus tests can be used for Ras–Raf-MEK-Erk, dabrafenib, vemurafenib, c-Jun, C-Fos, trametinib and sorafenib.
Can I send samples of specialized formulations and products for testing?
You are welcome to send us any substance for testing. We can also include additional natural substances in Onconomics Plus or Onconomics Extracts or additional chemotherapy drugs in the Onconomics test. We also offer combination tests, provided a doctor specifies the ratio of each component in the combination.
How reliable is a negative circulating tumour cell test result for patients who have no metastasis after surgery?
RGCC’s circulating tumour cell test has a negative predictive value of about 86%. This means our test will correctly return a true negative result 86% of the time.
Is ChemoSNiP a blood or buccal swab?
If you only need to order the ChemoSNiP test on its own, a swab is suitable. However, if you wish to order other tests in addition to the ChemoSNiP, a patient’s DNA will be isolated from their blood. In this case, a blood sample is sufficient for both tests, so there is no need for a swab.
Does ChemoSNiP examine the cause of mutation?
The ChemoSNiP test provides information about DNA mutations that can affect how humans develop cancer or respond to anti-cancer drugs and treatments.
Can Oncotrace differentiate between benign and malignant tumours?
Yes. The Oncotrace test provides information about the presence of circulating tumour cells, their concentration and immunophenotype, which may help identify their origin. It is used to provide guidance about a patient’s prognosis, and to help identify the primary tumour when this is unknown.
Can Immune-Frame tell if a patient’s immune system is suppressed as a result of cancer or if a suppressed immune system caused the cancer?
The Immune-Frame test focuses on the status of a patient’s immune system, based on their cellular markers and the level of cytokine proteins in the test sample.
How often should patients carry out a follow-up test?
This should be determined by your treatment plan.
How often will I need follow-up support tools?
Every three months for the first year, then every six months

Contact
SOL Partnership Sdn Bhd, 6th Floor, Lot 442, Wisma Hrih Lotus, Jalan Pahang, Setapak, 53000 Kuala Lumpur
Email: [email protected]